Zinc Assay Reagents
Reliable zinc assay for nutritional and clinical diagnostics.
Detects zinc deficiency or elevation with high accuracy in serum, plasma and urine samples.
High-Sensitivity Zinc Detection for Nutritional Deficiency and Disease Monitoring
Zinc is an essential trace element involved in numerous biological functions, particularly as a cofactor in various metalloenzymes. Zinc deficiency may lead to dermatitis, impaired taste and smell, poor wound healing, and growth disturbances. Causes of deficiency include malabsorption (e.g. liver or kidney disease), increased excretion, or chronic illness. Conversely, elevated levels may occur in conditions such as hemolytic anemia or eosinophilia.
The NAGASE zinc assay enables sensitive and accurate quantification of zinc in serum, plasma and urine. It features a wide linearity range (2–500 µg/dL), high repeatability, and minimal interference. The method shows strong correlation with ICP-MS and allows for direct urine testing without preprocessing.
NAGASE supplies zinc assay reagents in bulk for clinical diagnostics and OEM applications.
Applications
The zinc assay is suitable for a wide range of clinical and nutritional diagnostic settings.
- Assessment of zinc deficiency in patients with malabsorption or chronic disease
- Monitoring zinc levels in serum or urine for nutritional studies
- Diagnosis of taste or smell disorders linked to trace element deficiency
- Evaluation of delayed wound healing and growth disturbances
- Supportive testing in liver, kidney, or metabolic conditions
Features
The zinc assay provides precise, interference-resistant performance for serum, plasma and urine analysis.
- Wide linear range – 2 to 500 µg/dL for flexible testing across concentrations
- Excellent correlation – Matches well with ICP-MS reference method
- Minimal interference – Coexisting substances have low impact
- Direct urine measurement – No sample preparation required
- Long reagent stability – At least 8 weeks after opening
Intended Use
Our reagents are specifically designed for the in vitro-quantitative determination of Zinc in human serum, plasma and urine.
Principle of the Method
The concentration of zinc in a sample is measured by analyzing the change in absorbance that occurs when zinc (Zn2+) in the specimen forms a complex with 2-(5-bromo-2-pyridylazo)-5-(N-propyl-N-sulfopropylamin-o)phenol sodium salt (5-Br-PAPS), using a spectrophotometric analysis instrument.
Assay Procedure
Individual instrument applications are available on request.
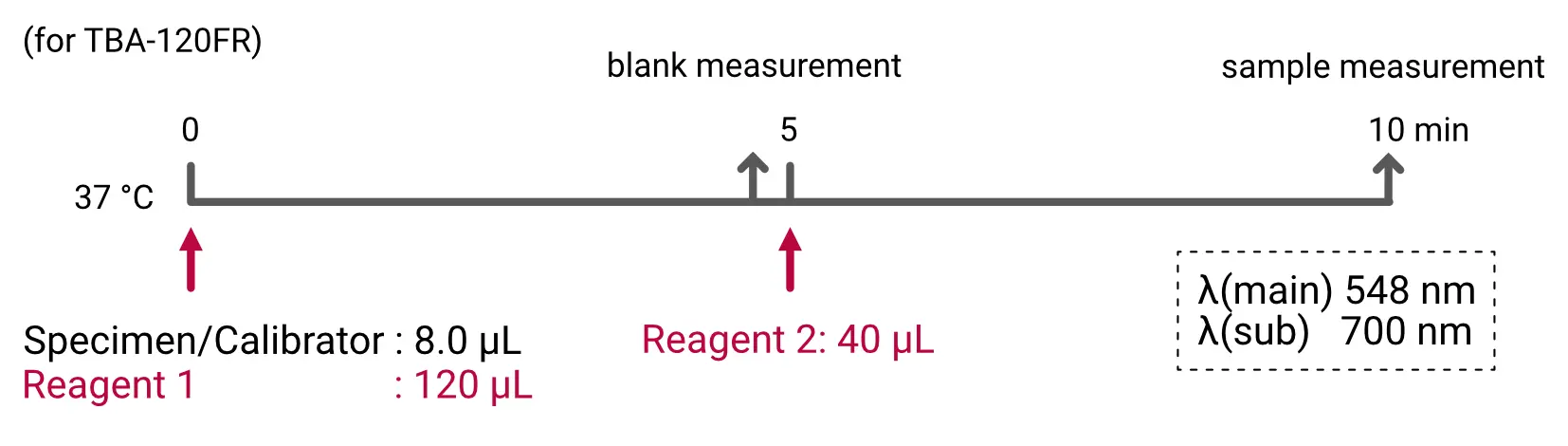
Assay Reagents, Storage and Stability
Reagent 1: Buffer
Reagent 2: 5-Br-PSAP
Both reagents are liquid, ready to use
Storage: 2-10 °C
Shelf Life: 12 months (before use)
Stability after Opening: at least 8 weeks at 2-10 °C
Performance Data
The following performance data were obtained on a TBA-120FR clinical analyzer.
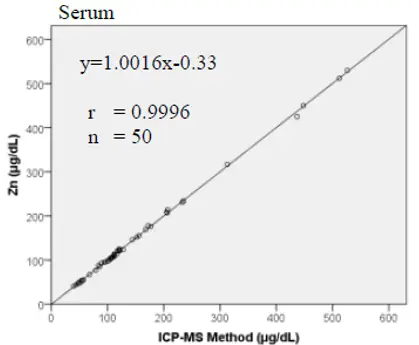
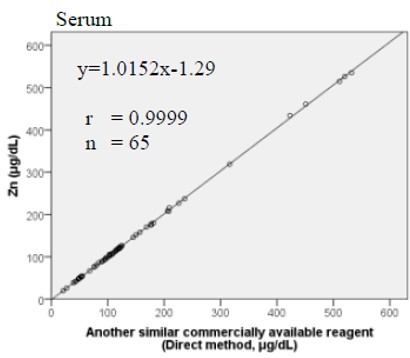
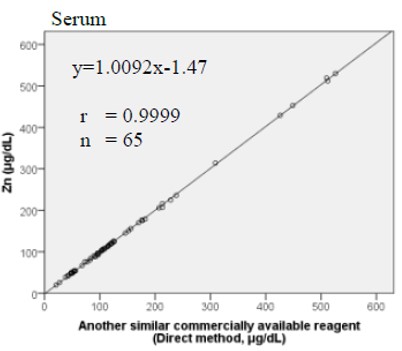
Method Comparison
Comparison studies were carried out using another similar commercially available method. The following results were obtained.
Sensitivity
The sensitivity was evaluated by reading the change in absorbance for purified water sample and standard solution with known concentrations. The results indicated that Zn showed little or no reagent drift on a zero sample.
Under the reaction condition described, 200 µg/dL standard solution gives a ΔABS of 0.04-0.24.
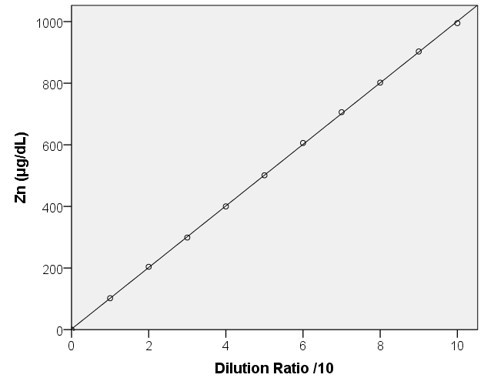
Linearity
Low Range
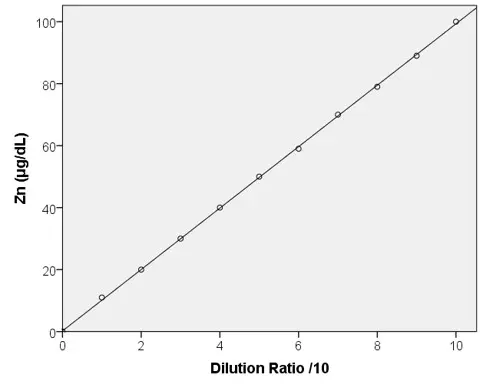
High Range
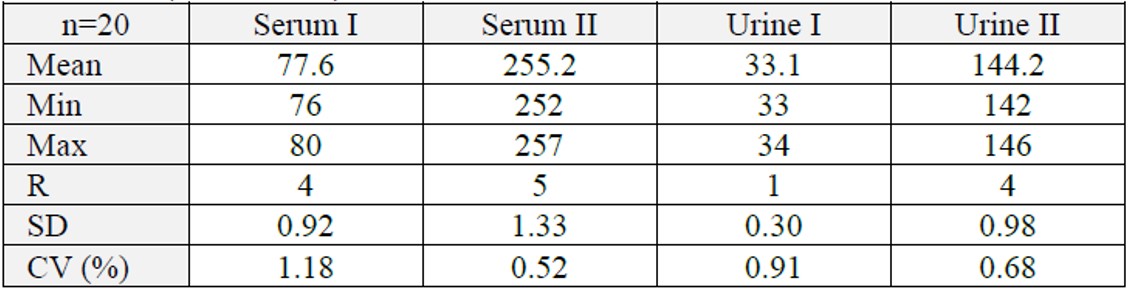
Precision (within-run)
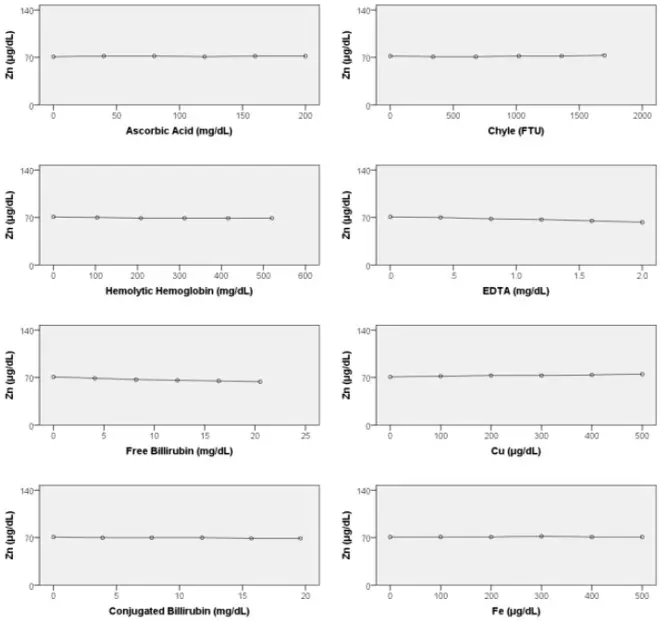
Interfering Substances