Pancreatic Amylase Assay Reagents
Highly specific alpha-amylase assay for improved pancreatitis diagnosis.
Offers enhanced sensitivity over total amylase detection by targeting pancreatic-origin alpha-amylase using an IFCC-transferrable method.
Targeted Diagnostic Solution for Accurate Pancreatic alpha-Amylase Testing
P-AMYLASE is a dedicated reagent system for the quantification of pancreatic alpha-amylase activity in human serum or plasma. While alpha-amylase is produced by several organs, including the pancreas and salivary glands, this assay is optimized to measure pancreatic-specific isoforms with higher clinical relevance in the diagnosis of acute pancreatitis.
Compared to total amylase testing, P-AMYLASE offers improved sensitivity and specificity, making it a more reliable tool in evaluating pancreatic injury. It is particularly useful for identifying pancreatitis, pancreatic trauma, or tumors, and may assist in monitoring late-stage pancreatic conditions or post-pancreatectomy recovery.
NAGASE supplies P-AMYLASE reagents for clinical laboratories, offering IFCC-compliant performance, high linearity, and consistent results.
Applications
P-AMYLASE is used to detect pancreatic alpha-amylase activity in diagnostic laboratories.
- Diagnosis of acute and chronic pancreatitis
- Evaluation of pancreatic trauma or tumors
- Monitoring of pancreatectomy recovery
- Assessment of late-stage pancreatic cancer
- Differentiation between pancreatic and salivary alpha-amylase sources
Features
P-AMYLASE offers high specificity and reliable performance for targeted clinical diagnostics.
- IFCC-transferrable method – Ensures standardization and global compatibility
- High linearity – Reliable measurement range from 3 to 2000 U/L
- Low coefficient of variation – Enables repeatable results across runs
- Reduced interference – Minimal cross-reactivity with coexisting substances
- Enhanced pancreatic specificity – Greater sensitivity than total amylase assays
Principle of the Method
This assay is used to measure pancreatic amylase (P-AMY) by an immuno-inhibition method. The anti-salivary amylase antibody reacts specifically with the salivary amylase (S-AMY) and inhibits its activity.
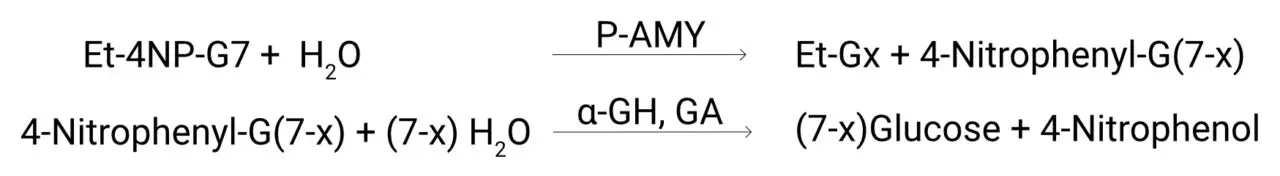
Ethylidene-4-NP-G7 (Et-4NP-G7) is hydrolyzed by pancreatic α-amylase to form 4,6-ethylidene-α-(1,4)-D-glucopyranosyl-Gx and 4-nitrophenyl-α-(1,4)-glucopyranosyl-G(7-x).
The 4-nitrophenyl-α-(1,4)-glucopyranosyl-G(7-x) is then hydrolyzed into glucose monomers and the 4-nitrophenol by α-glucosidase (α-GH) and glucoamylase (GA).
The resulting change in absorbance at 405 nm is proportional to the pancreatic α-amylase concentration in the sample. The use of the ethylidene prevents exo – enzymes from breaking down the substrate, so in the absence of α-amylase no color change is observed.
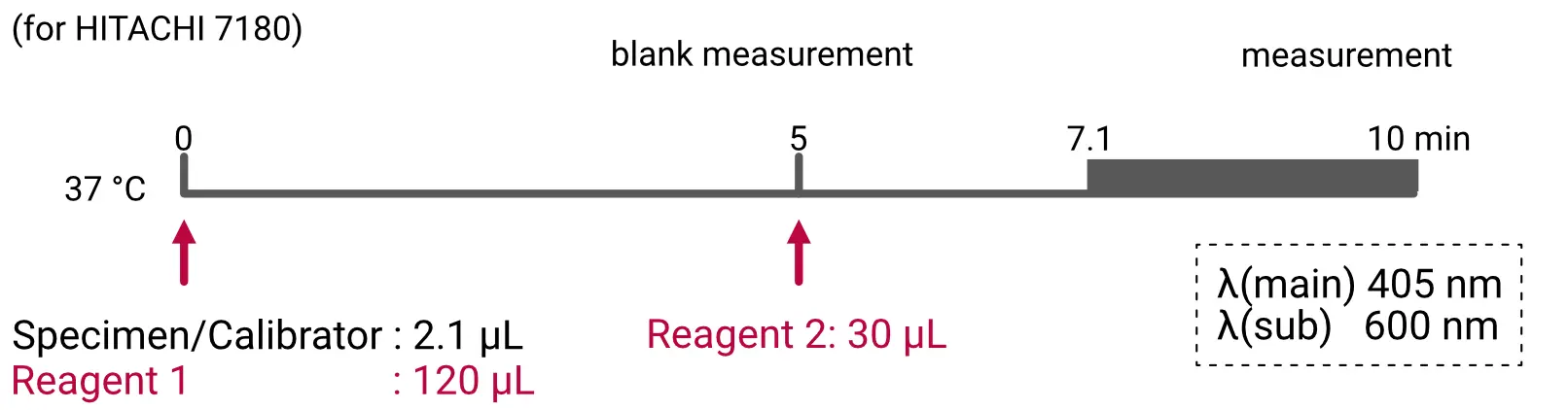
Assay Procedure
Individual instrument applications are available on request.
Assay Reagents, Storage and Stability
Reagent 1: Good’s buffer, Anti-Salivary Amylase Antibody (mouse monoclonal antibody), α-Glucosidase, Glucoamylase, Sodium chloride, Calcium acetate, pH 7.0 (25 °C)
Reagent 2: Good’s buffer, Et-4NP-G7, Sodium chloride, Calcium acetate, pH 7.0 (25 °C)
Both reagents are liquid, ready to use
Storage: 2-10 °C
Shelf Life: 12 months (before use)
Stability after Opening: at least 1 months at 2-10 °C
Performance Data
The following performance data were obtained on a Hitachi 7180 clinical analyzer.
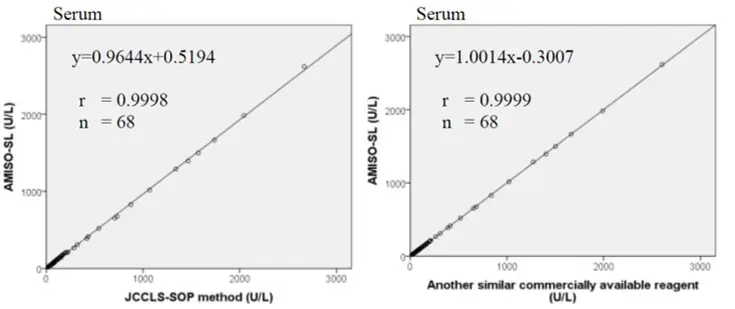
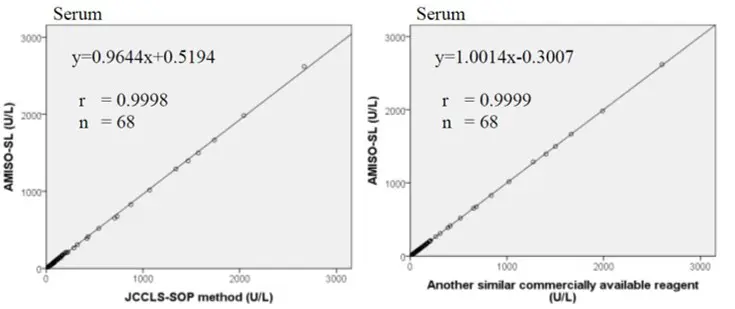
Method Comparison
Comparison studies were carried out using another similar commercially available method. The following results were obtained.
Sensitivity
The sensitivity was evaluated by reading the change in absorbance for purified water sample and serum samples with known concentrations. The results indicated that AMY-SL showed little or no reagent drift on a zero sample.
Under the reaction condition described, 321 U/L AMY activity gives a ΔABS/min of 0.011-0.030.
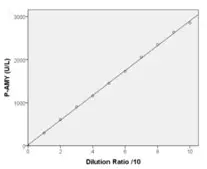
Linearity
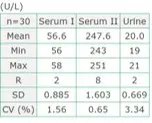
Precision (within-run)
Interfering Substances