Amylase Assay Reagents
High-performance alpha-amylase assay for clinical diagnostics.
Offers sensitive and accurate detection of alpha-amylase levels to support diagnosis of pancreatic and salivary gland conditions.
Comprehensive and Reliable Testing Solution for alpha-Amylase Activity
AMY-SL is a specialized reagent kit designed for the quantitative determination of alpha-amylase activity in human serum, plasma, or urine. As alpha-amylase is a key enzyme produced by the pancreas and salivary glands, its measurement provides essential diagnostic insights into pancreatic disorders such as acute pancreatitis, as well as salivary gland diseases including mumps and bacterial parotitis. The assay is also used in the differential diagnosis of abdominal conditions and exocrine pancreatic insufficiency.
The AMY-SL method is characterized by high sensitivity, low interference, and excellent reproducibility, making it suitable for both routine and emergency diagnostic workflows in clinical laboratories.
NAGASE supplies AMY-SL alpha-amylase assay reagents for medical laboratories and diagnostic manufacturers.
Applications
AMY-SL is widely used for clinical diagnostics involving pancreatic and salivary gland function.
- Diagnosis of acute pancreatitis and other pancreatic disorders
- Detection of mumps and bacterial parotitis
- Screening for salivary gland damage or disease
- Differential diagnosis of intra-abdominal conditions
- Monitoring of exocrine pancreatic insufficiency
Features
AMY-SL ensures accurate, efficient, and reproducible alpha-amylase testing in clinical settings.
- High sensitivity – Detects even low levels of alpha-amylase in biological samples
- Low interference – Stable readings even in complex clinical matrices
- Rapid reaction time – Suitable for emergency and high-throughput workflows
- Broad clinical applicability – Validated for serum, plasma, and urine samples
- Reliable reproducibility – Ensures consistent results across laboratories
Principle of the Method
This method is based on the recommendations of the International Federation of Clinical Chemistry (IFCC).
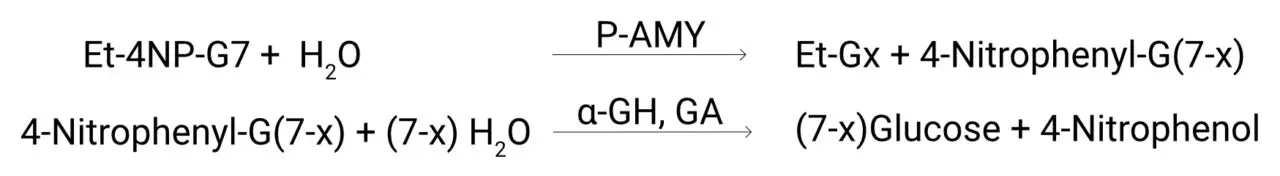
Ethylidene-4-NP-G7 (Et-4NP-G7) is hydrolyzed by α-amylase to form 4,6-ethylidene-α-(1,4)-D-glucopyranosyl-Gx and 4-nitrophenyl-α-(1,4)-glucopyranosyl-G(7-x). The 4-nitrophenyl-α-(1,4)-glucopyranosyl-G(7-x) is then hydrolyzed into glucose monomers and the 4-nitrophenol by α-glucosidase (α-GH) and glucoamylase (GA). The resulting change in absorbance at 405 nm is proportional to the α-amylase concentration in the sample.
The use of the ethylidene prevents exo – enzymes from breaking down the substrate, so in the absence of α-amylase no color change is observed.
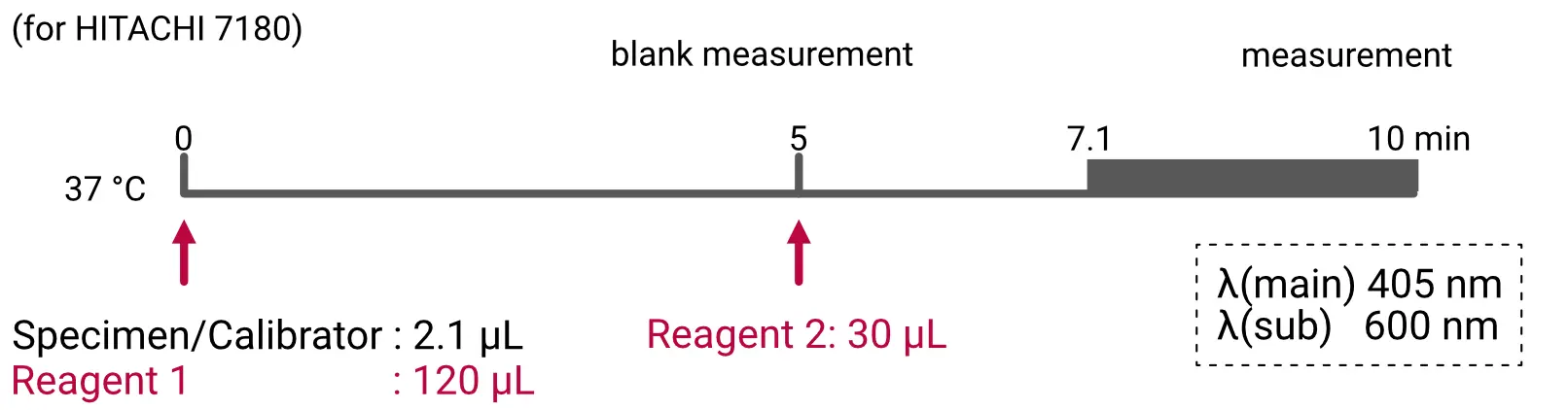
Assay Procedure
Individual instrument applications are available on request.
Assay Reagents, Storage and Stability
Reagent 1: Good’s buffer, α-Glucosidase, Glucoamylase, Sodium chloride, Calcium acetate, pH 7.0 (25 °C)
Reagent 2: Good’s buffer, Et-4NP-G7, Sodium chloride, Calcium acetate, pH 7.0 (25 °C)
Both reagents are liquid, ready to use
Storage: 2-10 °C
Shelf Life: 12 months (before use)
Stability after Opening: at least 1 months at 2-10 °C
Performance Data
The following performance data were obtained on a Hitachi 7180 clinical analyzer.
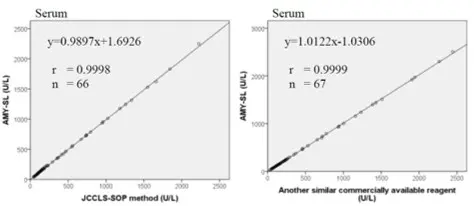
Method Comparison
Comparison studies were carried out using another similar commercially available method. The following results were obtained.
Sensitivity
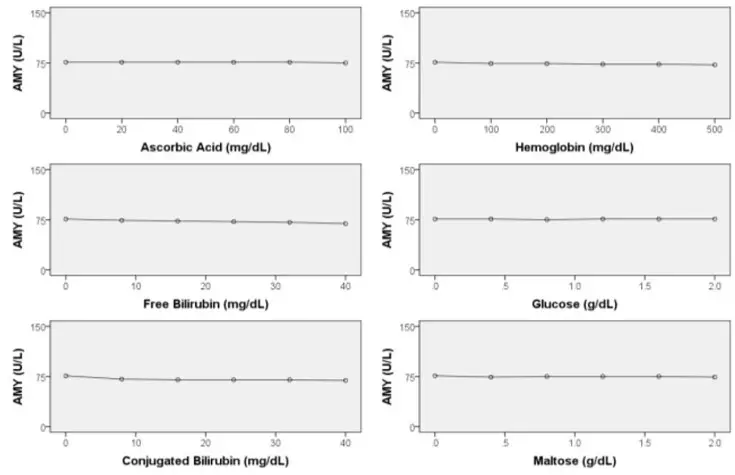
The sensitivity was evaluated by reading the change in absorbance for purified water sample and serum samples with known concentrations. The results indicated that AMY-SL showed little or no reagent drift on a zero sample.
Under the reaction condition described, 321 U/L AMY activity gives a ΔABS/min of 0.011-0.030.
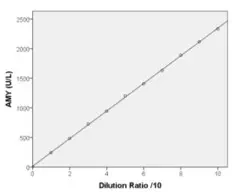
Linearity
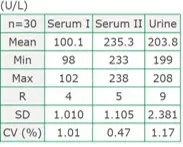
Precision (within-run)
Interfering Substances